Investors
We are pursuing investment to help fund additional clinical trials and build our portfolio of host response diagnostic assays.

Commercial strategy
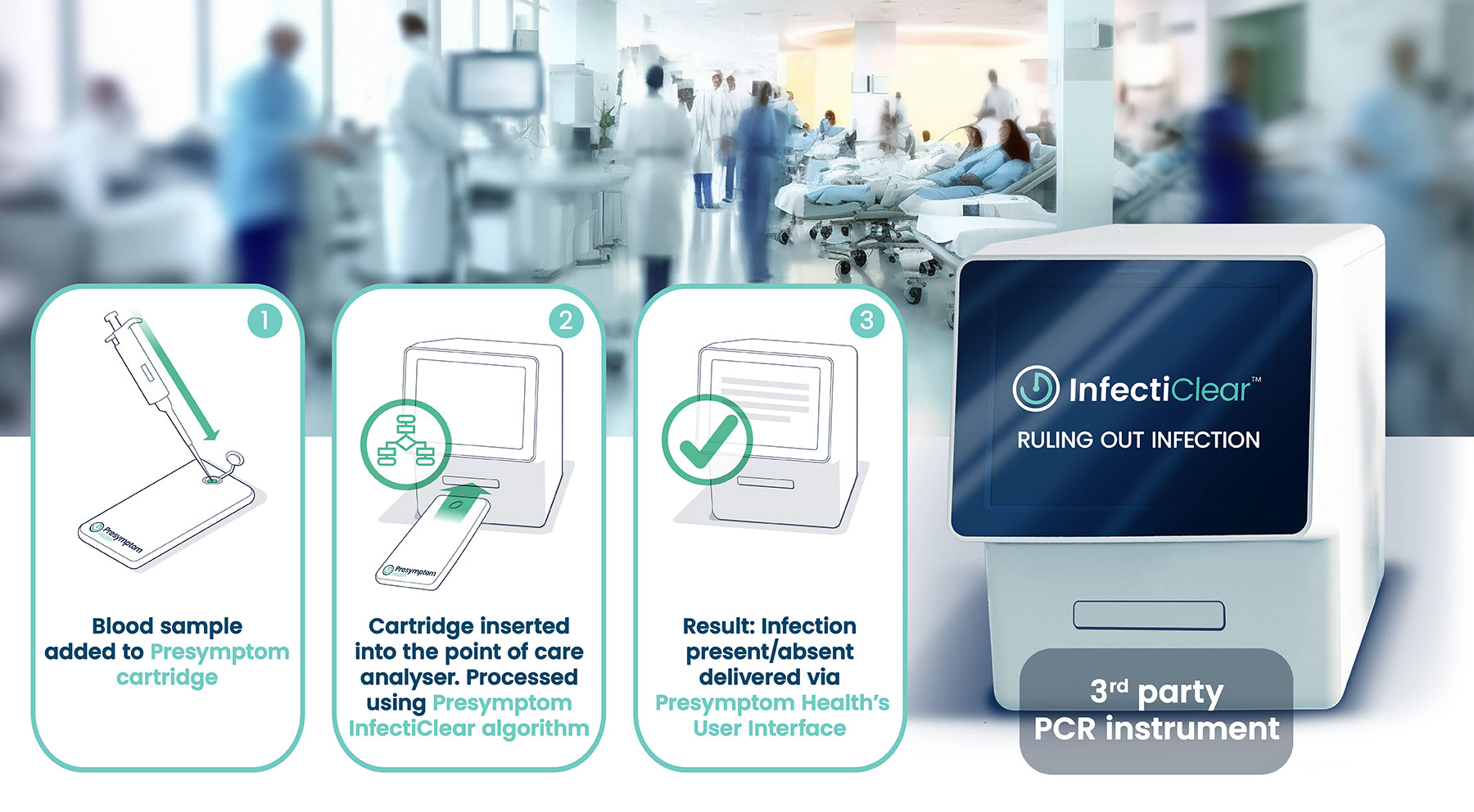
We plan a UK pilot launch of InfectiClear™ in 2025. We are also working on several second-generation clinical POC versions of the test designed to offer quicker turnaround times at the patient’s bedside.
Our development portfolio also includes assays to assess sepsis risk, which will complement InfectiClear™ in the management of patients with infection who are suspected of having sepsis. All products continue to be under active evaluation, and are being developed according to ISO 13485:2016 regulations for medical devices.
There is an estimated US$ 0.9 billion global market opportunity specifically for InfectiClear™ – excluding low- and middle-income countries – while the wider IVD market is considerably higher.1 We will continue to work with IDAP to prepare for commercialisation in the UK in 2025, which holds a 5-10 % share of the global IVD market, before launching in the US in 2028 (35 % market share) and finally in the EU (20-25 %).1
Our investors







