Host response diagnostics
Host response diagnostics
Our technology investigates the patient – rather than the pathogen – allowing us to rule out infection or sepsis well before current methods. This is made possible through years of clinical development and validation, generating unique data sets through which we have ‘trained’ our assays using advanced machine learning techniques.
Detecting infection status earlier
Most current diagnostic tests aim to detect the pathogen itself, but our technology focuses on the patient’s (host) immune response to the presence of pathogens.
This can help to:
Our host response prognostic test – InfectiClear™
InfectiClear™ is an accurate, reliable rule-out blood test that confirms the absence of infection before symptoms would appear, and prior to current diagnostic techniques. This highly sensitive ‘pathogen agnostic’ approach investigates immune system changes that occur at the onset of infection, significantly reducing time to diagnosis and providing clinicians with the information they need – within clinical guidelines – to pursue other diagnostic investigations.
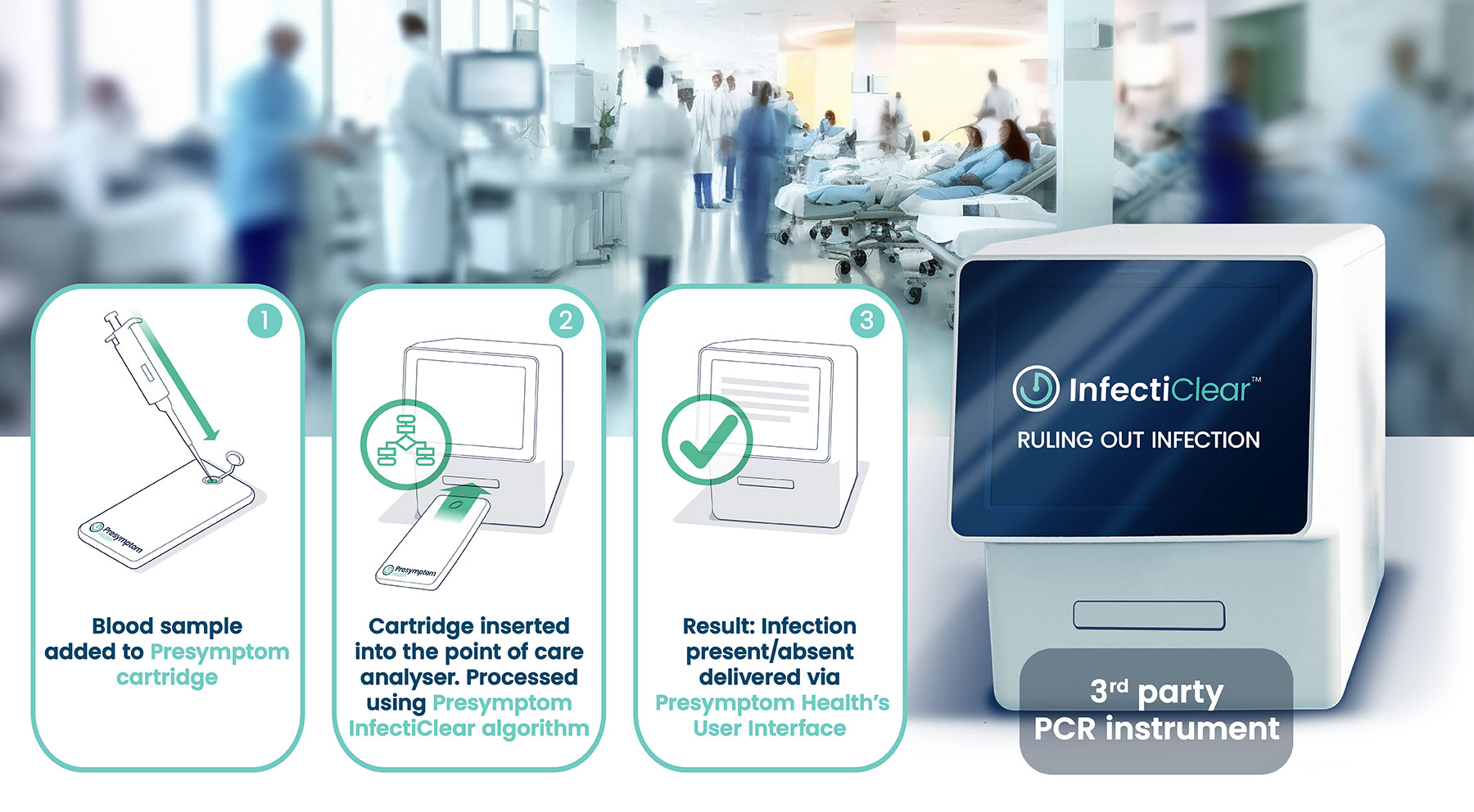
Next generation technology
InfectiClear™ is one of eight technologies selected for the Innovative Devices Access Pathway (IDAP) pilot. This initiative is designed to accelerate the development of new medical devices that address unmet clinical needs in the NHS, helping to ensure they are available to patients and healthcare professionals at the earliest opportunity.
With only a handful of companies selected, inclusion in IDAP is a major milestone for the company, as it is providing access to various experts – including scientific, regulatory and commercial personnel – to further guide the development of InfectiClear™, navigate the regulatory landscape, and help in the final stages of commercialisation.
Innovation for the diagnosis of infection and sepsis
InfectiClear™ is scheduled for release in 2025, and will be available with a user-friendly, local or cloud-based software package that generates an infection score indicating the likelihood of infection. This will provide clinicians with the confidence to withhold, delay or reduce antibiotics in the event of a low-risk score.
Key benefits:
Wealth of clinical data

Discovery
Our technology is based on a 10-year foundational discovery study conducted by the Defence Science and Technology Laboratory (Dstl), involving over 4,000 patients who underwent surgery.2 Nearly 100,000 samples were collected sequentially with associated clinical metadata – starting just before surgery and extending to one week after – with some patients developing postoperative infection and sepsis.
We identified specific genes that were expressed differently between this cohort and those with an uncomplicated recovery, and applied proprietary machine learning and AI to this data, leading to the discovery of a set of biomarkers that can reliably predict the onset of infection and sepsis prior to clinical presentation and diagnosis.
Verification and validation
The discovery phase was followed by a number of studies to verify and validate our findings, involving more than 1,000 additional patients, for a combined total of 5,500 patients across all of our research to date. These included verification studies on seven NIH and EMBL-EBI in silico cohorts encompassing 513 sepsis and control patients, demonstrating high accuracy in all viral and bacterial datasets.
This was followed by participation in the EU-funded SEPTIMET study, which had the aim of ‘improving the speed, accuracy and reproducibility of diagnostic tests for the identification and treatment of sepsis’. Again, we demonstrated the ability to clearly distinguish sepsis patients from control groups. Presymptom Health also analysed COVID-19 samples from the GenOMICC study to verify infection status in these patients.
Further studies and analyses to validate these findings are in progress or planned, and will add to the wealth of data behind InfectiClear™:
- 1
PRECISION, which now has completed enrollment, is a multi-centre observational cohort study involving nearly 500 patients across nine NHS trusts. It is designed to distinguish patients with uncomplicated infection from those with either sepsis or no infection (control).
- 2
PLUTO is a study aimed at improving pre-operative risk stratification and enabling earlier recognition of surgical complications.
- 3
PRECISION 2.0 is planned to start in mid-2025 across three NHS hospitals and will recruit 350 patients.














